COVID-19 Vaccine Information
Boosters and Vaccines Available
The Gerald L. Ignace Indian Health Center has vaccines available, by appointment only. Offering to those eligible, including ages 6 month to 4 years old. Call 414-383-9526 to schedule.
To see if you are eligible visit: CDC Website or Wisconsin DHS Website
Get Your At Home Test Kits
Every home in the U.S. is eligible to order 4 free at-home COVID-19 tests. The tests are completely free. Orders will usually ship in 7-12 days.
-
The tests available for order:
-
Are rapid antigen at-home tests, not PCR
-
Can be taken anywhere
-
Give results within 30 minutes (no lab drop-off required)
-
Work whether or not you have COVID-19 symptoms
-
Work whether or not you are up to date on your COVID-19 vaccines
-
Are also referred to as self-tests or over-the-counter (OTC) tests
-
Where Can I Get Vaccinated/Booster?
GLIIHC continues to offer the vaccine on an appointment basis to those who wish to get vaccinated and or receive boosters. Call us at 414-383-9526 to schedule yours.
You can also utilize www.vaccines.gov or www.healthymke.com as resources for finding vaccines available nearest to you. We will of course keep you up to date on when we are able to offer vaccines to the expanded age group. We appreciate your inquiries, and urge all to get the Covid19 vaccine when eligible. #vacciNATION
Health care professional clinical materials were updated to reflect changes in the ACIP recommendations for booster dose guidance and were posted last night, including:
Standing orders for each vaccine product
- Pfizer 12 years of age and older: Pfizer-BioNTech COVID-19 Vaccine: 12 Years of Age and Older • Standing Orders for Administering Vaccine (cdc.gov)
- Moderna: Moderna COVID-19 Vaccine: Standing Orders for Administering Vaccine to Persons 18 Years of Age and Older (cdc.gov)
- Novavax: Novavax COVID-19 Vaccine: Standing Orders for Administering Vaccine to Persons 12 Years of Age and Older (cdc.gov)
- Pre-vaccination checklist: S. COVID-19 Vaccine Product Information | CDC
The updated versions of the Preparation and Administration Summaries for each vaccine product, COVID-19 Quick Reference Guide for Healthcare Professionals and Summary of Interim Clinical Considerations are with the web team and should be posted soon.
Preparation and administration summaries:
- Pfizer 12 years of age and older: Pfizer-BioNTech COVID-19 Vaccine Preparation and Administration Summary (cdc.gov)
- Moderna: Moderna COVID-19 Vaccine: Vaccine Preparation and Administration Summary (cdc.gov)
- COVID-19 Vaccine Quick Reference Guide for Healthcare Professionals: S. COVID-19 Vaccine Product Information | CDC
- Summary Document for Interim Clinical Considerations: Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC
Below are some highlights from an interview with an infectious disease MD (Dr. Sagreti) at Rush University in Chicago. Please use the link below to reference the entire article:
https://www.rush.edu/news/covid-19-vaccines-what-you-need-know
Q: How effective are the Pfizer and Moderna vaccines?
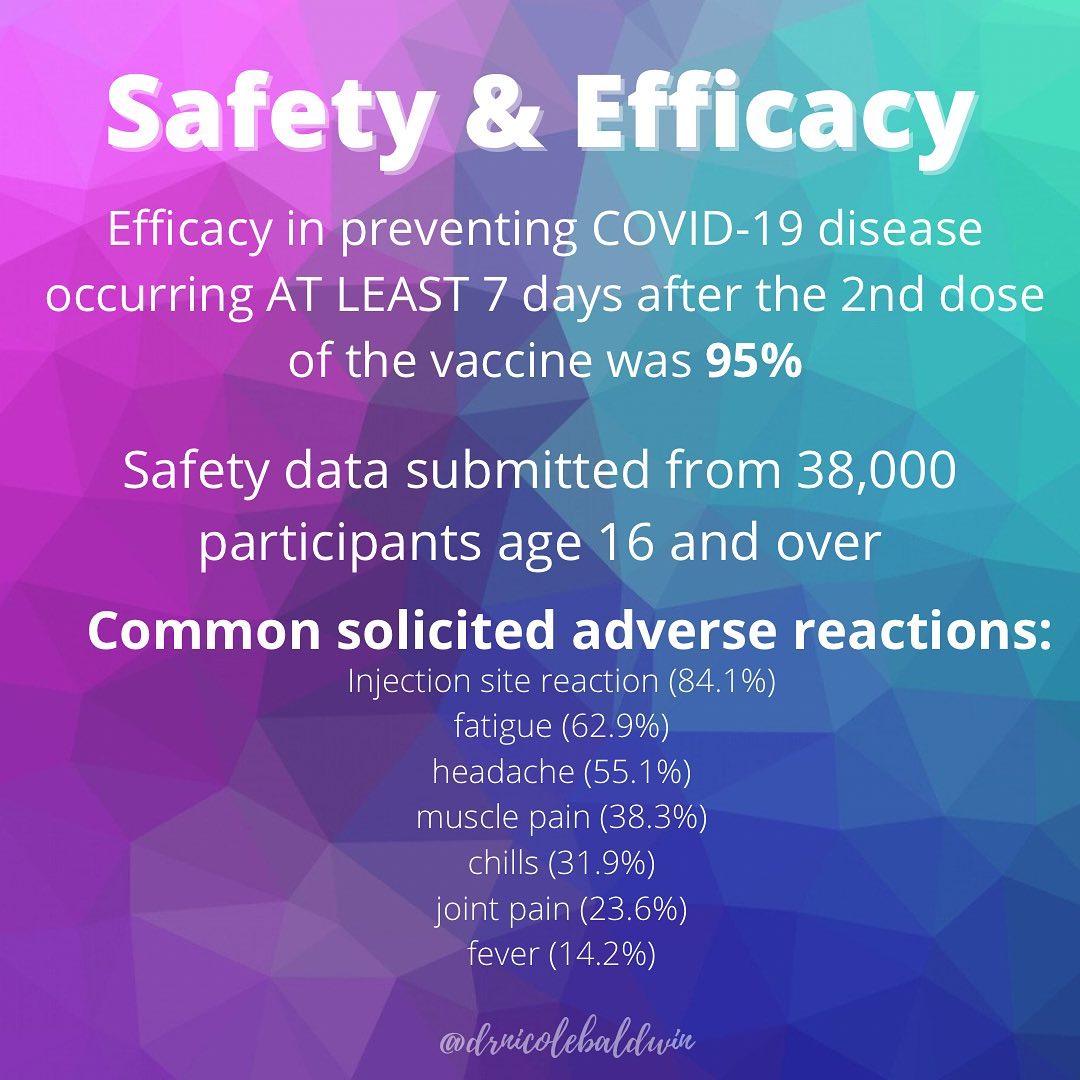
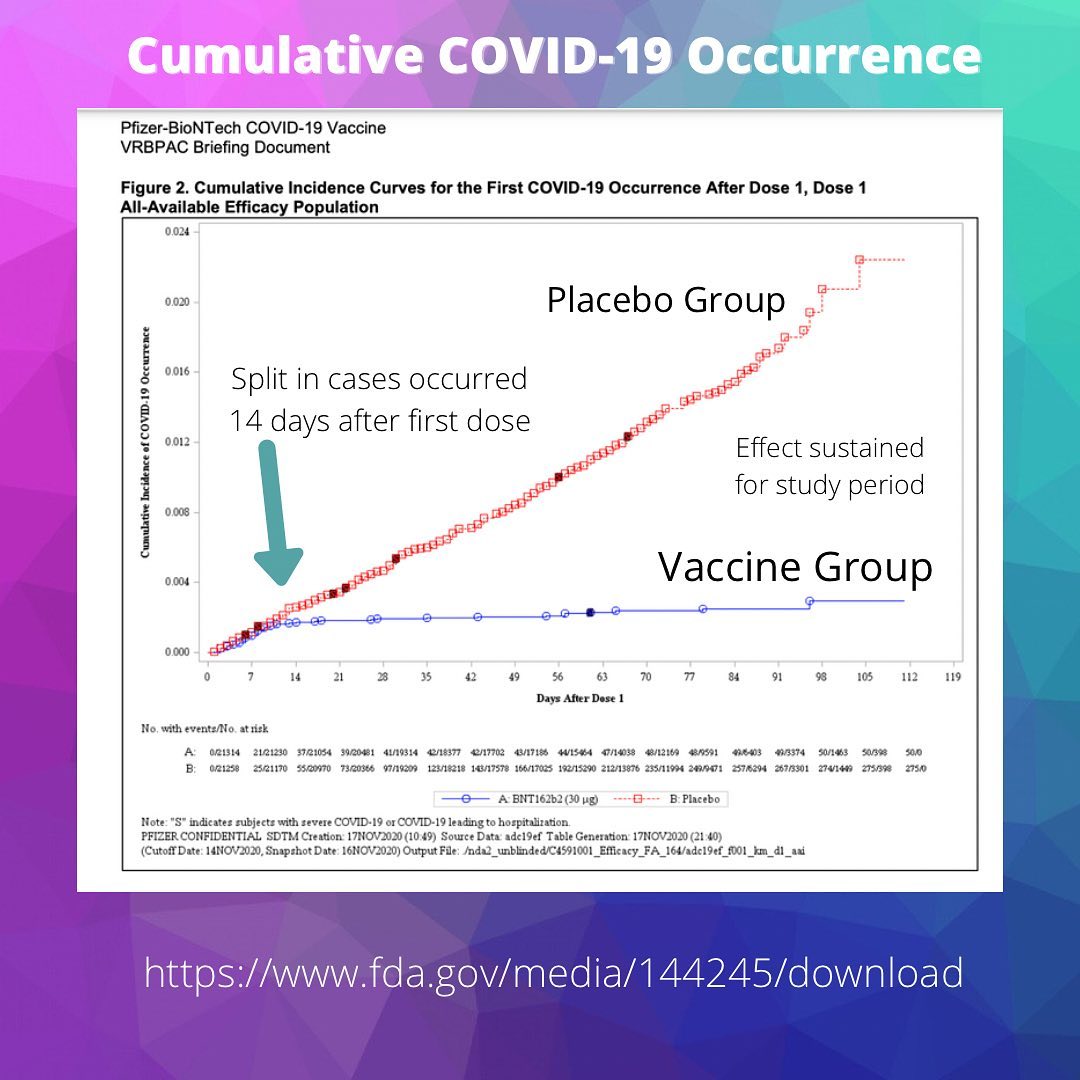
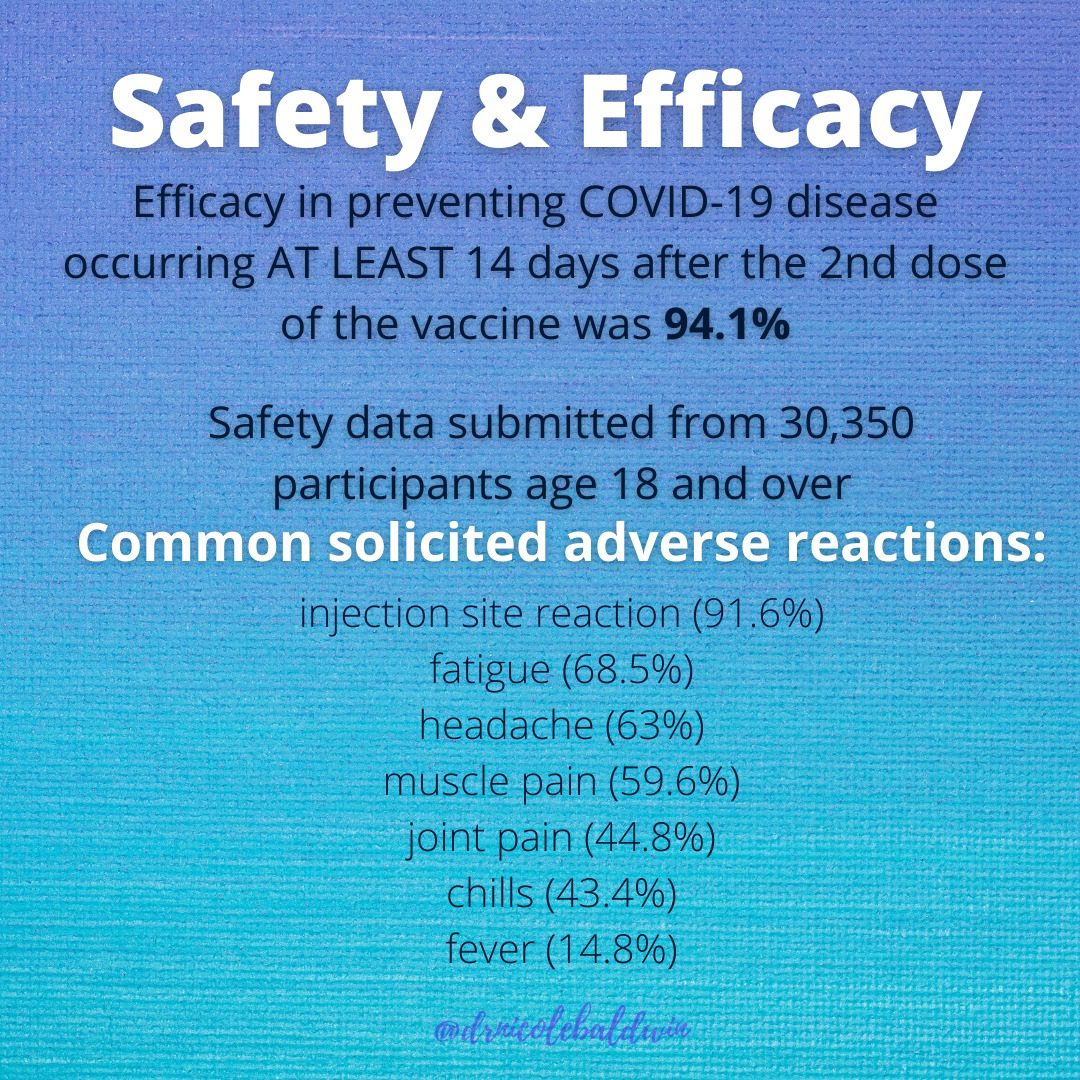
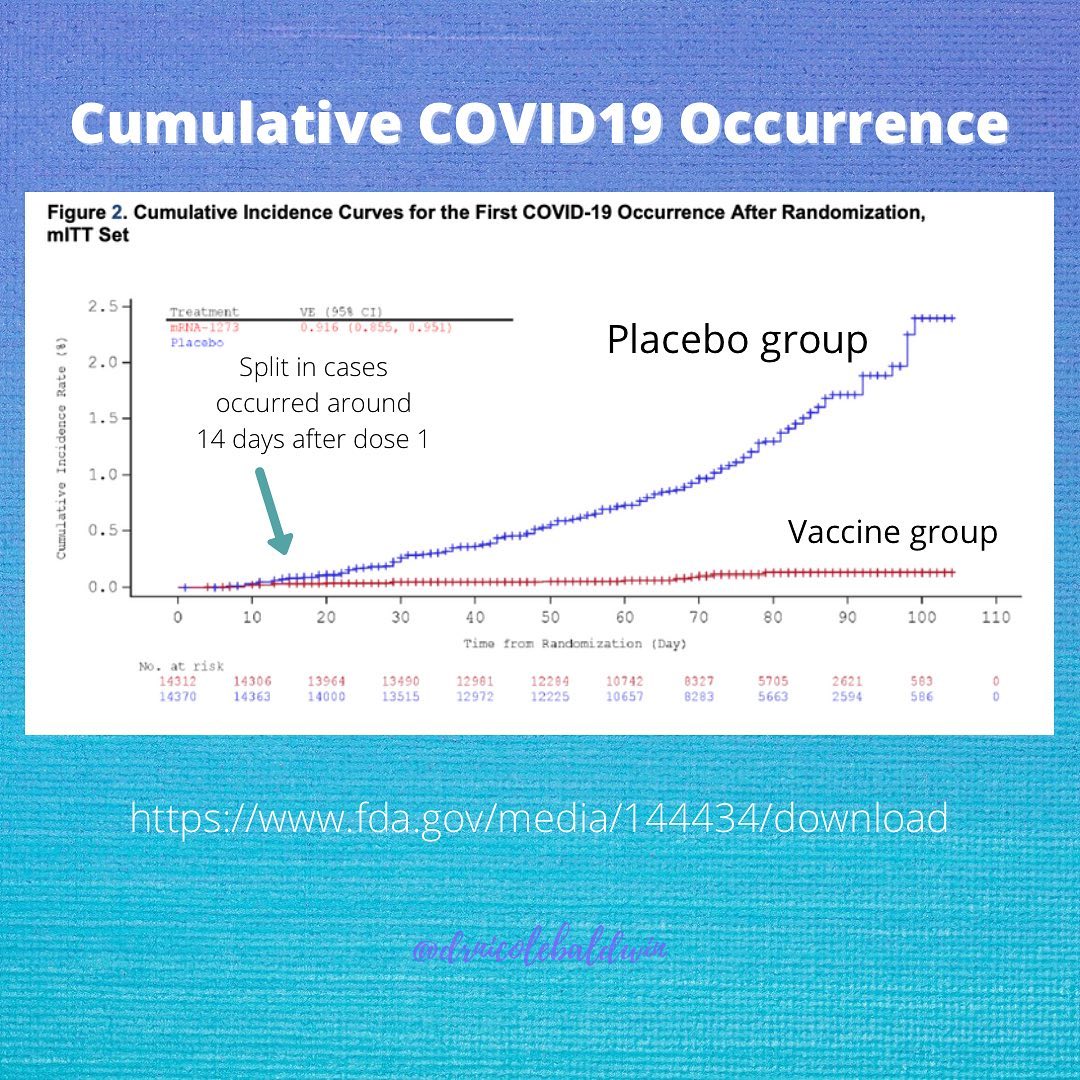
Segreti: Both vaccines showed around 95% effectiveness, which means that in the placebo-controlled trial, 95% of those who got COVID-19 received the placebo; only 5% of those who received the vaccine got COVID-19. The Pfizer trial included about 44,000 volunteers, and the Moderna trial had more than 30,000.
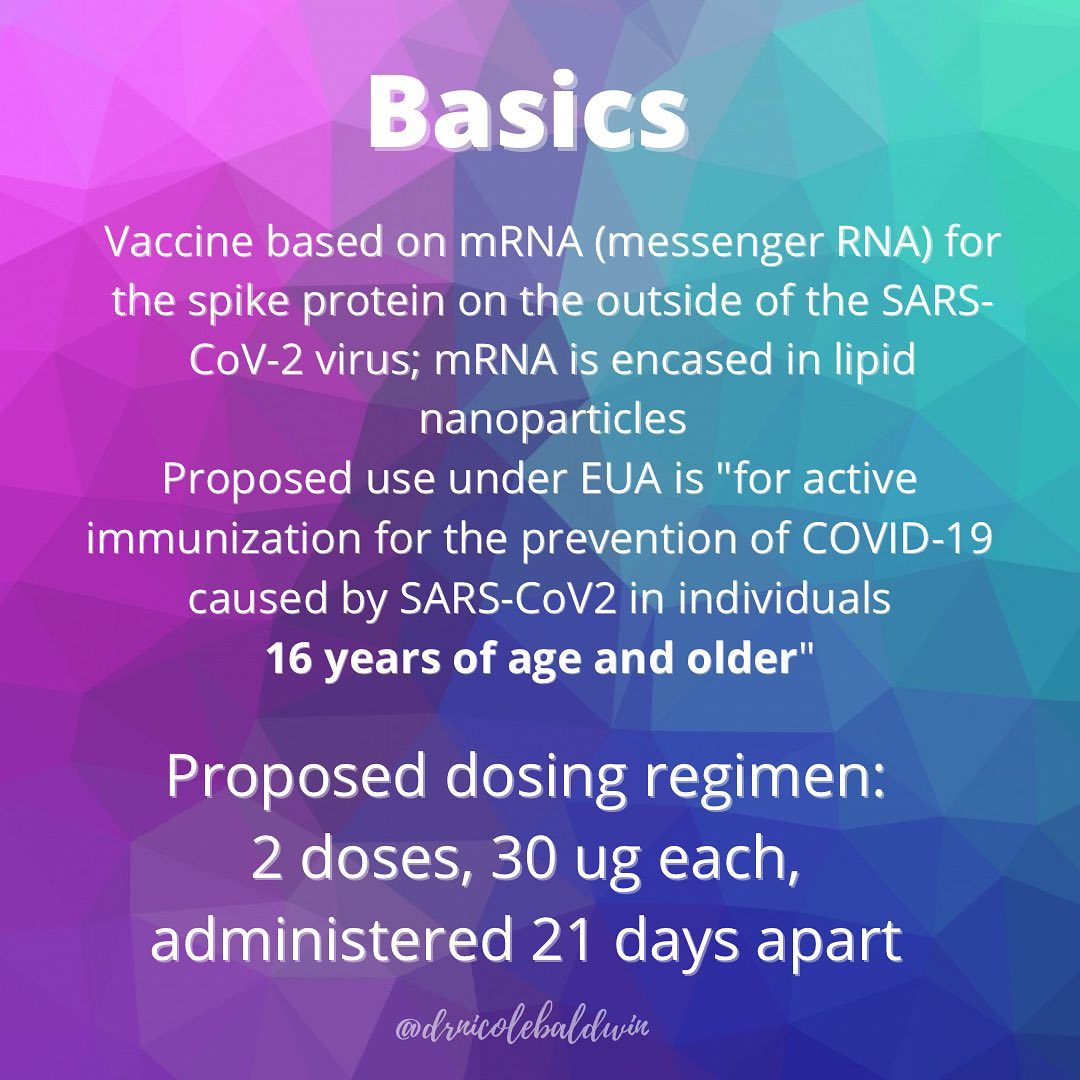
Both vaccines require two doses: Pfizer’s 21 days apart and Moderna’s 28 days apart. And both use the same messenger RNA technology to deliver the vaccine, which is great from a production standpoint because the vaccines can be manufactured and distributed a lot more quickly.
Q: How does the vaccine work? What is messenger RNA?
Segreti: mRNA, or messenger RNA, is a molecule that carries the information cells use to produce different proteins: Think of it as a blueprint. The vaccines harmlessly mimic the virus’ ability to trigger the body’s immune responses to infections. The Pfizer and Moderna vaccines include mRNA from the coronavirus “spike” protein — which is what enables the virus to infect cells.
Q: Can the vaccine give you COVID-19?
Segreti: As with other vaccines, COVID-19 vaccines cannot give you the virus. They do not contain a live virus.
Q: Are the vaccines safe?
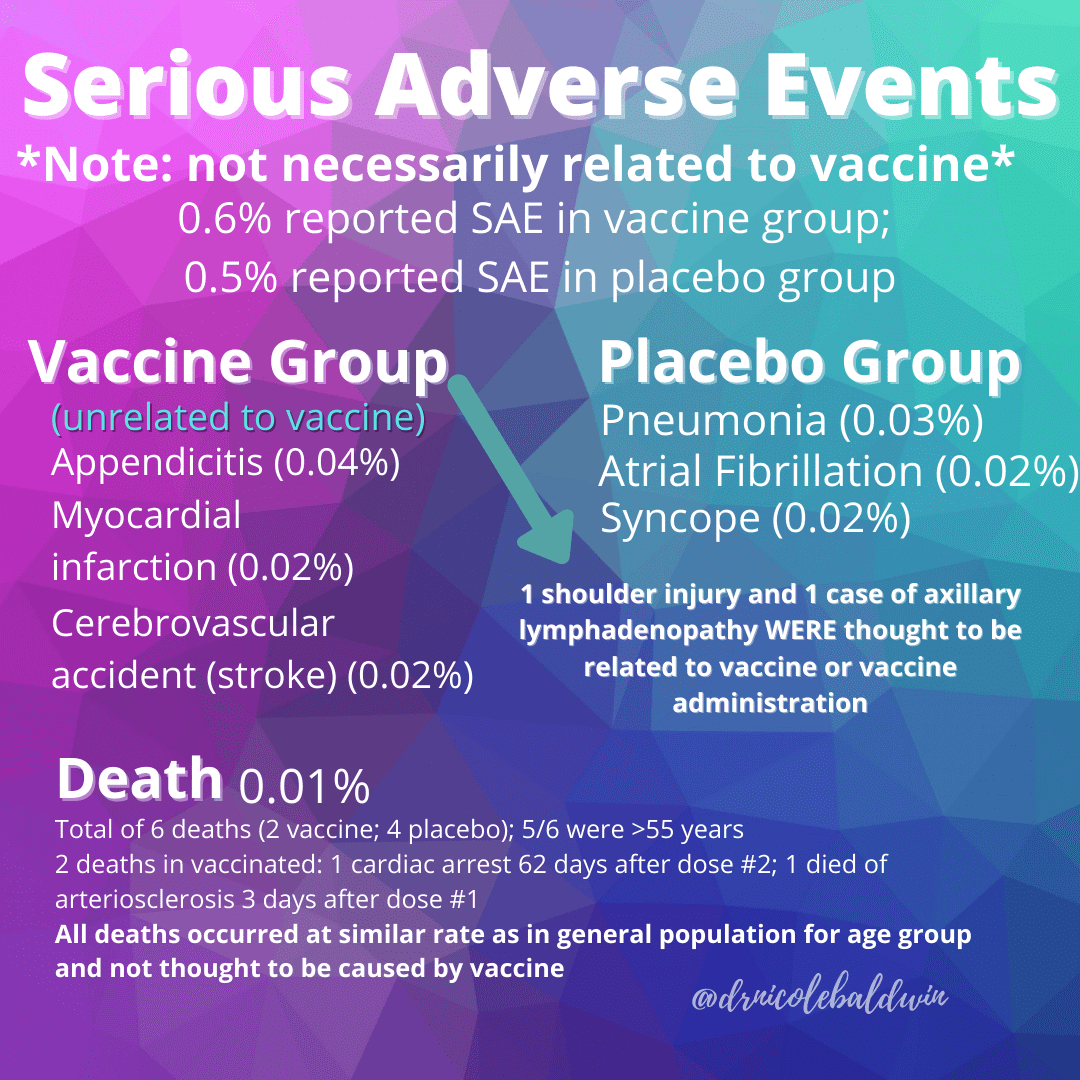
Segreti: Both the Pfizer and Moderna vaccines seem to be very safe and most people have been able to tolerate them pretty well. Neither of their phase 3 clinical trials was discontinued or even temporarily halted due to any adverse event, and fewer than 2% of recipients of these vaccines developed severe fevers of 102.2°F to 104°F.
The independent board that conducted the interim analysis of Moderna’s huge trial found that severe side effects included fatigue in 9.7% of participants, muscle pain in 8.9%, joint pain in 5.2% and headache in 4.5%. For the Pfizer vaccine, the numbers were lower: Severe side effects included fatigue (3.8%) and headache (2%).
Q: Will the COVID-19 vaccine be needed annually, similar to the flu vaccine?
Segreti: We don’t know yet how long the vaccine will last, so we don’t know if or how often people will need to be vaccinated again. We will make decisions about the need for future vaccinations based on what we learn going forward.
Q: Will people who had COVID-19 already be able to get the vaccine? Do they need to get it?
Segreti: As of right now, we don’t think there’s any reason not to get vaccinated, but we will clarify that issue with the local health departments.
If you had COVID-19 and recovered from it, you probably have some antibodies to the virus that causes the disease. But we don’t entirely know what that means as far as your protection against reinfection. While we have not seen too many people get reinfected with COVID within six months of becoming sick, we don’t know how long any immunity might last. So pending guidance from the local health departments, our recommendation will likely be to get vaccinated even if you previously had COVID-19.
Please view this informative 6 minute YouTube video of Dr. Fauci, from the NIH. It discusses the production and safety of the vaccine, as I know this was topic of concern among staff. It also gives a nice little overview of how Covid-19 actually affects our body. Minute 4 discusses how Covid-19 clinical trials moved along more quickly than the other historical “normal” timelines.
Native Strong Town Hall on COVID-19 Pandemic w/ Dr. Lyle Ignace
COVID-19 Pfizer Vaccine Trial Details

COVID-19 Moderna Vaccine Trial Details

COVID-19 Vaccine Information Sources
CDC (Center for Disease Control and Prevention)
NIH (Nation Institute of Health)